The Number of Hydrogen Atoms in 6nh3 Is ____.
Log in for more information. Updated 8312020 73817 PM.

Total Number Of Atoms Present In 34 G Of Nh3 Is
So there will be 18 hydrogen atoms in 6NH₃.

. 6 3 9 18. The number of hydrogen atoms in 6NH3 is. If an electron is gained the atom has a negative charge.
1 Show answers Another question on Biology. Asked 8312020 51421 PM. The number of hydrogen atoms in a molecule of 6NH3 is 18.
S Score 1itoldusoPoints 7090 Log in for more information. In 3 molecules of H2O which is 3H2O there are 6 hydrogen atoms and 3 oxygen atoms. The number of hydrogen atoms in 6NH3 is _____.
Score 1 itolduso Points 7090. Asked 8312020 51421 PM. The number of hydrogen atoms in 6NH3 is.
The number of hydrogen atoms in a molecule of 6NH3 is 18. If an electron is gained the atom has a _____ charge. There are 18 hydrogen atoms in 6 molecules of NH3 or ammonia.
This answer has been confirmed as correct and helpful. Area that electrons are found around an atomic nucleus. The number of hydrogen atoms in 6NH3 is ____.
The number of hydrogen atoms in a molecule of 6NH3 is 18. The number of hydrogen atoms in 6NH3 is ____. Ask Your Own Homework Question.
The number of hydrogen atoms in 6nh3 is. What is the number of Hydrogen Atoms in the molecules of 6NH3. Because 6 x 3 18 6NH3 6 Nitrogen Hydrogen 3 So that would be 6 times 3 hydrogen.
3 is only for HHydrogen. The number of hydrogen atoms in 6NH3 is - 1257372. A new substance formed by the combination of two or more different atoms.
Updated 8312020 73817 PM. The smallest complete unit of a compound or diatomic gas. The number of the hydrogen atoms in a molecule of 6NH3 is 18.
Updated 266 days ago10212020 100115 PM. Asked 266 days ago10212020 53903 PM. 6 3 9 18.
Log in for more information. You multiply the coefficient of 3 times the subscript of each element. To determine the number of hydrogen atoms in this molecule what would you do is simply take the coefficient and multiply it to the subscript present for hydrogen which is 3.
Ascientist is conducting an investigation that involves water silver carbon dioxide and oxygen gas which group of statements best describes all of the materials he is using in the investigation. The number of hydrogen atoms in 6NH3 is 18 while the number of of Nitrogen atoms is 6. Get 1-on-1 help from an expert tutor now.
For finding the number of hydrogen atoms we should multiply 6 with 3 So 63 18. The number of hydrogen atoms in a molecule of 6NH3 is 18. The final result would be 6 X 3 or 18.
What is the number and type of atom in 3 H2O. When there is no subscript it is assumed to be 1. Up to 25 cash back What is the number of.
The number of hydrogen atoms in 6NH3 is ____.

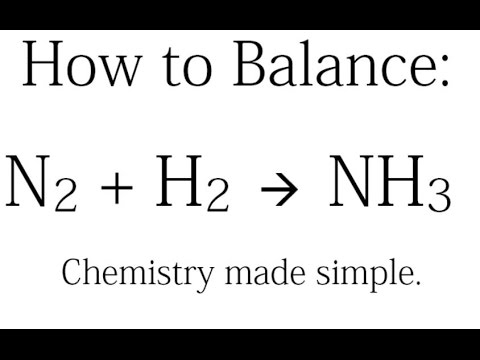
How To Find The Number Of Atoms In Nh3 Ammonia Youtube

How To Find The Number Of Atoms In Nh3 Ammonia Youtube


Comments
Post a Comment